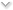Seen on a recent menu. They reprinted it to include this information; they could have reprinted with the actual higher prices, and if they ever intend to drop the surcharge they'll have to reprint again, so the only motivation seems to be to disguise the price hike/keep it out of customers' minds as they are ordering.
Wednesday, May 31, 2023
Tuesday, May 23, 2023
Mexican flag and "taste of Mexico" not enough to deceive reasonable consumers about non-Mexican origin, 2d Cir rules
Hardy v. Olé Mexican Foods, Inc., 2023 WL 3577867, No. 22-1805 (2d Cir. May 22, 2023) (per curiam)
There was a CD
Cal case raising the same “false Mexican origin” claims with a different
result. Hardy alleged that defendant’s La Banderita tortilla products violated
the NY GBL by deceiving consumers into believing the products were made in Mexico,
not the US. The court of appeals affirmed the dismissal of the complaint.
Hardy had standing for the product he purchased, and class
standing for three unpurchased products that made the same allegedly false
claims that led to a price premium.
“On the La Banderita Products, a graphic resembling the
Mexican flag (but with corn stalks instead of the coat of arms of Mexico)
figures prominently in the center of the packaging and sets the green-white-red
color scheme of the packaging.” The edges of the packaging may also display a
smaller version of the flag graphic – this time with a white bull replacing the
white segment of the flag – and the phrase “A Taste of Mexico!” On the back of
the packaging there is a La Banderita logo and the flag graphic, followed by a
“Nutrition Facts” table, heating instructions, and barcode. The bottom-left
corner includes graphics stating that the products are “MANUFACTURED BY: OLÉ
MEXICAN FOODS, INC. NORCROSS, GA 30071” and “MADE IN U.S.A.”
In the context of the whole package, this was not likely to
mislead a reasonable consumer. While the front of package features “may
encourage consumers to draw associations with Mexico and promote the belief
that the products contain Mexican-style flavors and ingredients, no reasonable
consumer would construe these elements to be an affirmative representation that
the La Banderita Products were in fact manufactured in Mexico.” This was
especially true given the conspicuous back-of-package statement. There is no
rule that information on the back of a package is always irrelevant.
While a small-print ingredient list cannot “cure” front-label representations
that are otherwise highly deceptive because “reasonable consumers expect that
the ingredient list contains more detailed information about the product that
confirms other representations on the packaging,” that isn’t necessarily true
outside the context of nutritional labels. It’s not true here, where “the
front-side packaging makes no express representations as to the origin of the
La Banderita Products, while the back of the packaging unambiguously notes
where the products were ‘made’ and ‘manufactured.’”
Monday, May 22, 2023
court: there's no right to jury trial when seeking only injunction/disgorgement in false advertising case
Bluegreen Vacations Unlimited, Inc. v. Timeshare Lawyers P.A., 2023 WL 3510374, No. 20-24681-Civ-Scola (S.D. Fla. May 17, 2023)
The court grants these timeshare plaintiffs’ motion for a
bench trial, ruling that the Seventh Amendment doesn’t guarantee a jury trial
in a false advertising case where the plaintiffs seek only equitable remedies.
I presume that the defendants think that a jury might be more sympathetic to
their unclean hands-type arguments, and they also argued they'd wasted a bunch of time and resources preparing for a jury; the court notes that “a significant portion
of the Defendants’ opposition highlights what is perhaps best referred to as
Bluegreen’s persistent gamesmanship in this and related cases.”
The issue of “whether
a right to a jury trial exists turns on whether the claims were historically
cognizable at law or considered equitable.” This requires comparing the
statutory action to 18th-century actions, then examining the remedy sought; the
latter is the more important consideration. The Eleventh Circuit previously held
that a plaintiff in a trademark infringement suit under the Lanham Act is not
entitled to a jury trial when it seeks only disgorgement of the defendant’s
profits in lieu of actual damages. Hard Candy, Ltd. Liab. Co. v. Anastasia
Beverly Hills, Inc., 921 F.3d 1343, 1348 (11th Cir. 2019).
Although this was a false advertising case with no obvious
18th-century analogue, the court found Hard Candy dispositive. The
nature of the relief requested—disgorgement and an injunction—was the
determining factor. This also applied to plaintiffs’ tortious interference and
civil conspiracy claims, even though both causes of action were traditionally
cognizable at law.
alleged price bait-and-switch with large "processing fee" suffices to plead Lanham Act false advertising
New Vision Unlimited, LLC v. Glasses USA, Inc., 2023 WL 3535386, No. 22-22534-Civ-Scola (S.D. Fla. May 18, 2023)
New Vision, an eye-care professional and provider of optical
goods and services, including contact lens fittings and contact lens sales with
seven brick-and-mortar locations and two websites, brought a putative class
action against four online contact-lens retailers for Lanham Act false advertising.
The court denied a motion to dismiss.
Defendants promote their lenses through Google and other
search engines, advertising low prices that are allegedly “far lower” than the
prices New Vision and other “honest” retailers advertise. Consumers of contact
lenses are allegedly very price conscious and thus this works, but, New Vision
alleged, “only after a customer has filled out online forms, providing details
about her prescriptions, contact information for her doctor (to verify her
prescription), and certain biographical and payment information, do the
Defendants reveal there is a ‘processing fee’ added to the order” of about
twice the initially advertised cost. This final cost is allegedly “about the
same as the prices honest retailers such as Plaintiff advertise, because this
is the true market price.”
Although New Vision alleged that the processing fee was
hidden enough that, “many times,” “consumers do not even notice that the total
amount they are being charged for the order is about the same as what they
would have paid if they bought from [New Vision],” screenshots in the complaint
show the processing fee presented in plain type, in normal-size font, in the
middle of the secure checkout page. (Those things are not necessarily
incompatible, given that consumers might think there’s nothing more than
ministerial stuff to do to check out, especially given autofill. I wonder what
discovery may show about whether consumers ever complain afterwards.) New
Vision alleged that, even if consumers do notice the fee, sunk costs keep them
at the sites, and they may also mistakenly believe that all contact-lens
retailers charge similar “processing fees.”
Defendants argued that their ads were literally true because
consumers can actually purchase one box of the advertised contact lenses for
the published price, and any conceivable deception was cured by the full
disclosure of the added processing fees prior to final purchase.
Defendants supplied screenshots from each of their websites,
showing that no processing fee is added to a consumer’s purchase when only one
box is selected. But first, there was no way of knowing whether these current
offerings were the same as those during the period at issue in the complaint.
Second, the allegations of the complaint were that the typical orders were
twice as much as advertised, which this didn’t refute. Third, some of the ads
were at least capable of being false even under this interpretation. E.g., one
of the Contact Lens King ads in the complaint offers a “90 Pack” for “Only
$26.22/box.” “While it may be possible to interpret this, as the Defendants
urge, to mean that Contact Lens King will in fact honor the $26.22 price if a
customer’s purchase is limited to one box, that is certainly not the only
interpretation. … [A] reasonable interpretation would be that the advertised
$26.22 price is per box, regardless of how many boxes are ordered.”
Nor did disclosure prior to final checkout solve the
problem, given the sunk costs allegations that “the complained of fees are not
revealed until deep into the purchasing process or at least not until the
consumers have already been lured away.” The court also rejected the argument
that, because the complained-of practices were allegedly commonplace, they
weren’t actionable. There is consumer protection even in the market for lemons!
Along with literal falsity, misleadingness was also
plausible. Even if “consumers won’t notice a processing fee that doubles the
cost” was problematic as an allegation, New Vision offered other deceptiveness
theories, based on bait and switch advertising/sunk costs.
Materiality was also plausible. Defendants argued that, if
consumers didn’t notice the near doubling of the price, then the advertised
prices couldn’t have been material; and if they did notice but buy anyway, that
also showed immateriality. But “the role the initial alleged price deception
played in driving consumers away from New Vision and towards the Defendants”
was also relevant. “It is not the materiality of the processing fees that is at
issue here but, rather, it is the materiality of the deceptive prices published
in the Defendants’ ads that drives consumers to the Defendants’ websites and
then ultimately influences their final purchasing decisions.”
Defendant-favorable cases, including resort fee cases, were distinguishable as
involving more/earlier disclosure (and mostly ignore the sunk costs problem
anyway). Defendants “improperly framed their materiality analysis, focusing
only on the consumers’ ultimate decisions to go through with their purchases of
the Defendants lenses, without any consideration of the separate deceptive
advertisement that got them there.”
Although some of the injury allegations were conclusory, New
Vision alleged sufficient harm. It described a “typical scenario,” in which one
of New Vision’s patients asks for a price for a particular product, compares
New Vision’s price to defendants’ ads, “complains that Plaintiff’s store is
‘overcharging’ and ‘too expensive,’ ” and, despite staff’s attempts to explain,
leaves without buying and ends up “believing that Plaintiff’s prices are not
competitive across the board.” The court was willing to accept that New Vision
was attesting to actual sales it has lost in the manner it describes as a
“typical scenario.”
Great balls of fire: lawsuit over malt sold looking nearly identical to whisky can continue
McKay v. Sazerac Co., 23-cv-00522-EMC (N.D. Cal. May 17, 2023)
The court rejected Sazerac’s motion to dismiss McKay’s usual
California statutory and common law claims on behalf of a putative class
based on Sazerac’s labeling and marketing of mini bottles of Fireball malt
beverages, which look almost the same as Fireball Whisky.
 |
| image from complaint |
 |
| Fireball Malt back |
Did you know that Fireball Whisky has been sold since 1989 and is the fifth best-selling spirit in the United States? It’s Canadian whisky—a distilled spirit—with added cinnamon syrup and sweeteners, and is 66 proof (33%) alcohol by volume (ABV). It’s sold in many sizes, from 1.75L bottles to 50ML mini bottles. The label reads “Cinnamon Whisky.”
Fireball Malt debuted in 2020. It is a malt or wine-based
beverage with added cinnamon syrup, sweeteners, and whisky flavors, and results
in 33 proof (16.5%) alcohol by volume (ABV). It has no whisky and is only sold
in 50ML mini bottles. The label reads “Cinnamon” and, in smaller font, “Malt
Beverage With Natural Whisky & Other Flavors and Carmel Color.”
Because it’s not hard liquor, it can be sold in more
locations, such as gas stations and convenience stores.
The court rejected Sazerac’s argument that it was protected
by the safe harbor doctrine, which allows conduct that the legislature has
specifically considered and permitted. Here, the Alcohol and Tobacco Tax and
Trade Bureau granted a certificate of label approval (COLA) covering Fireball
Malt. The regs say that a COLA may not be issued if the label contains “any
statement or representation, irrespective of falsity, that is misleading to
consumers as to the age, origin, identity, or other characteristics of the malt
beverage” or if any label statement “directly creates a misleading impression
or if it does so indirectly through ambiguity, omission, inference, or by the addition
of irrelevant, scientific, or technical matter.” But “neither of these
provisions clearly permit the type of representations made by Sazerac in the
Fireball Malt label.” “To forestall an action under the unfair competition law,
another provision must actually ‘bar’ the action or clearly permit the
conduct.”
The claims here didn’t turn solely on Sazerac’s failure to
use font of the minimum size required by a regulation or disclose added
flavoring as required by another regulation. “Rather, it is the combination of
the labeling and appearance that creates confusion between the whisky and malt
bottles. Plaintiff’s claims are predicated on a comparative analysis of the two
products in question. Importantly, there is no evidence that the TTB
regulations address any sort of comparative analysis with other labels, or test
for consumer confusion.”
As another court reasoned, “COLAs are too ‘informal’ to be
considered regulations having the force of law. Compared to the ‘rigorous’
approval process for prescription-drug labels, the TTB process ‘hinges on self
reporting’ and reflects only the representations made to it by the distributor,
not an endorsement of those claims.”
The court also found fraud adequately pled, including for
the statutory claims. The complaint plausibly alleged affirmative
misleadingness given that the two products’ labels were “substantially the
same,” including the brand name “Fireball,” Sazerac’s fire- breathing dragon
logo, the words “RED HOT,” the same color scheme, burnt edge, font and labeling,
and the words “CINNAMON.” Fireball Malt bore the ambiguous descriptor “Malt
Beverage With Natural Whisky & Other Flavors and Carmel Color” and was
packaged in a 1.7-oz, shot-size bottle “common for hard liquor.” The court
rejected Sazerac’s argument that the other descriptors on the label make plain
that Fireball Malt is a malt beverage, not a whisky, citing to the language of
“Malt Beverage,” “ALC 16.5% BY VOL,” and “WHAT YOU HAVE HERE TASTES LIKE SMOOTH
WHISKY WITH A FIERY KICK OF RED HOT CINNAMON” located on the back of the
product.
Tasting “like” whisky didn’t clearly preclude a reasonable
interpretation that it was or included whisky. “It is also not clear that
consumers would carefully scrutinize the tiny print of ‘Malt Beverage’ and ‘ALC
16.5% BY VOL’ in light of the other flashier language and design of the packaging.”
Ordinary consumers don’t have “an obligation to conduct extensive linguistic
analysis on their grocery shopping runs.”
Sazerac further argued that McKay should have known that the
products he bought in a gas station were not whisky because gas stations are
not licensed to sell hard liquor. But a reasonable consumer “could well be
unaware of which establishments hold which liquor license types. He may not
know that a gas station store can sell malt but not whisky. This is especially
so if he is making a quick roadside purchase. He might also assume that the
store is not complying with the terms of its liquor license.” The complaint
even alleged that “[o]ne radio personality who saw a huge Fireball display in
front of the cash register at a gas station wondered if ‘that specific store
was doing something they’re not supposed to be doing’ by selling ‘cinnamon
flavored whiskey!!’” Nor would a reasonable consumer need to compare his
purchase with other beverages in the vicinity to assess whether the store sold
only beer/wine and malt. Sazerac argued that it has structured its distribution
such that Fireball Whisky and Fireball Malt and never both placed in the same
store. “But the reasonable consumer may not be aware of Sazerac’s distribution
practices.”
McKay also successfully alleged concealment under the CLRA,
FAL, and UCL. An actionable “omission must be contrary to a representation
actually made by the defendant, or an omission of a fact the defendant was obliged
to disclose.” Such an obligation arises when (1) “the omission was material,”
(2) the omission relates to a fact that is “central to the product’s function,”
and (3) one of four special circumstances exists, including partial
representations that are misleading because some other fact has not been
disclosed. That was alleged here.
It was too early to tell whether equitable claims had to be
dismissed because legal relief was sufficient; McKay also had standing to seek
injunctive relief.
Second Circuit signals some minimal flexibility on Polaroid analysis in another strip club false endorsement case
Souza v. Exotic Island Enters., Inc., 2023 WL 3556053, No. 21-2149-cv, --- F.4th ---- (2d Cir. May 19, 2023)
Whereas the timeshare false advertising cases might be
making law largely applicable to other timeshare cases, what’s going on in the
strip club advertising cases might have somewhat broader implications. (Both
sets feature lots of cases against lots of defendants; the strip club cases
seem to be more geographically dispersed, which may also contribute.)
Appellants, current and former professional models, appealed
their summary judgment loss on a variety of claims arising from the use of
their images in social media posts promoting a “gentlemen’s club” operated by EIE.
The court of appeals affirmed, including on the district court’s decision not
to exercise supplemental jurisdiction over remaining state claims.
The basic undisputed allegation was that EIE, through a
third-party vendor, used plaintiffs’ images without their permission in social
media posts promoting its club.
The district court concluded that plaintiffs’ false
endorsement claims were foreclosed by Electra v. 59 Murray Enters., Inc., 987
F.3d 233 (2d Cir. 2021); their false advertising claims were founded upon
injury that either fell outside the zone of interests protected by the Lanham
Act, or that was unsubstantiated by the record; and the bulk of their state-law
right of publicity claims were barred by New York’s one-year statute of
limitations for such claims.
In substantially identical declarations, plaintiffs testified
that because they “rely on [their] professional reputation[s] to book modeling
and advertising jobs,” their reputations are “critical” to the opportunities
they are offered, and they therefore “have spent considerable time and energy”
protecting and policing their images and reputations, and carefully negotiating
their modeling fees based on “informed assessment[s]” of any given job’s effect
on their brands. Plaintiffs had “varying levels of success and visibility in
their modeling careers.” Several had appeared in magazines, advertising
campaigns, television episodes, and films. Some were former Playboy Playmates.
Their highest yearly modeling earnings range from around $18,300 to around
$107,000. Their social media footprints range from several thousand to a few
million followers. They had “fleeting” links to New York, and most no longer
work as full-time models.
From 2014-2018, the posts at issue used revealing photos of
the plaintiffs against ad copy linked thematically to the visual, e.g., a picture
of one plaintiff “in an apparent school uniform that included a short plaid
skirt, captioned: ‘Friday Oct 17th SEXY SCHOOL GIRL PARTY! No Cover For Ladies
That Wear A Sexy School Girl Skirt[.] All Our Dancers Will Be Wearing Short
Plaid Skirts!’”
In response to interrogatories, plaintiffs couldn’t identify
specific jobs or work lost as a result of the posts. Instead, they responded
that “prospective clients have no duty to disclose to the model their reasoning
for why the model was denied an endorsement opportunity. It is also a widely
known fact that on the outset of creating a highly coveted endorsement deal,
clients and/or their advertising agencies will conduct due diligence of models
in advance of contacting a model to discuss an endorsement opportunity.” Thus,
they argued, they couldn’t know their losses.
Is likely confusion an issue of fact or one of law? “[A]lthough
our stance may have wobbled over the years, recent cases have solidified our
view that, ‘[i]nsofar as the determination of whether one of the Polaroid
factors favors one party or another involves a legal judgment – which it often
does – we must review that determination de novo.’” Thus, the overall standard
of review is de novo: whether confusion is likely is a matter of law.
False endorsement assesses the Polaroid factors.
Here, the district court correctly held that strength of the mark favored
defendants. Recognizability is the bottom line for strength of a mark. As
previously held, “misappropriation of a completely anonymous face could not
form the basis for a false endorsement claim.” Although inherent
distinctiveness is also relevant to strength generally, there was no argument
that plaintiffs’ marks were inherently distinctive. And the court noted that
inherent distinctiveness as a concept was “more awkward to apply when it
effectively interrogates how much one human being does, or does not, physically
resemble another…. The usual criteria for inherent distinctiveness … have
little application here.”
For false endorsement, the “mark” in question “is the
identity of the purported endorser herself.” But
an endorser’s face and body fall
nowhere on the familiar spectrum from “arbitrary” to “generic”; their identity
inherently is their mark. And where any face or figure regarded as “attractive,”
will do, notwithstanding the anonymity of the actual person whose face or
figure is depicted (and the negligible endorsement value derived from that
actual person’s connection to the product being sold), the unauthorized use of
that person’s image may invade rights granted by other statutes or common law
sources, but creates no risk of consumer confusion as conceived under the
Lanham Act.
Some limits on the Polaroid spectrum! The other way
to say it is that indicia of identity are descriptive, but this works too.
Anyway, “recognizability thus serves the purposes of trademark law in the false
endorsement context.”
The district court correctly evaluated the relevant evidence
of recognizability. It didn’t abuse its discretion in excluding plaintiffs’
proffered expert testimony as unreliable, and there was next to nothing left
without that. A district court “should only exclude [expert] evidence if the
flaw is large enough that the expert lacks good grounds for his or her
conclusions.” The proffered expert’s survey of 812 respondents “who had
patronized ... a ‘Bikini Bar/Gentlemen’s Club/Strip Club’ in the past two
years,” wasn’t reliable because of methodological problems: The study lacked a
control group; had an “overly inclusive” approach to recognition; and failed to
show respondents several plaintiffs’ full faces “(which, the court noted,
somehow did not appear to have meaningfully affected respondents’ professed
ability to recognize the Plaintiffs who were pictured).” The overly inclusive
bit was because recognizability is about whether consumers would actually
assume sponsorship or approval and not just mere appearance in an ad; finding
an image “vaguely familiar” or having “in some manner” seen the plaintiff
before taking the survey wasn’t helpful in answering that question.
The remaining evidence was insufficient:
This consisted of (1) vague and
conclusory (and identical) written testimony from Plaintiffs that each had
“achieved celebrity status and fame” and that “[o]n any given day, regardless
of where I’m at, I am recognized by complete strangers and my fans who follow
me on social media,” and (2) evidence of Plaintiffs’ relatively modest modeling
income, professional prominence, and social media footprints, which the
district court determined (in a finding Plaintiffs do not contest on appeal) to
be, at best, comparable to evidence the Electra panel had deemed
insufficient to establish a strong mark.
After excluding more of the expert’s testimony, actual
confusion also favored defendants. That testimony claimed that: (1) 62% of
respondents agreed with the statement that “[a]ll the women shown in these ads
have some affiliation, connection or association with those clubs in whose ad
they appear”; (2) 75% agreed that “[a]ll of the women in these ads have agreed
to sponsor, endorse or promote the club represented in these ads”; and (3) 76%
agreed that “[a]ll of the women in the ads approve of the use of their image in
those Club advertisements in which they appear.”
But this part of the survey required respondents to give
their impressions of all the images, rather than differentiating among
different images and/or plaintiffs, and the survey neither provided respondents
with a “don’t know” option nor instructed them “not to guess.” This wasn’t
abuse of discretion (even though other district judges might have done otherwise).
Without that, there was no “meaningful” evidence of actual
confusion. At deposition, one of defendants’ witnesses was asked if the fact
that one of the posts at issue included the phrase “our girls” would cause a
“reasonable customer” to believe “well, they’re saying our girls; this is one
of the girls who is going to be there.” He responded: “That’s up to them. I’m
not – some may, some may not. It’s the individual’s discretion if they choose
to believe what they see.” This was his own subjective perception of the post’s
effect on consumers; it “may be probative of his state of mind, but nothing
about [his] speculative answer to a speculative question establishes that any
actual consumer was actually confused.”
In addition, even if consumers were “hoodwinked” as
described, “thus giving rise to a plausible deceptive trade practices claim,” that’s
not false endorsement:
A generic misconception that the
anonymous, unrecognized models in the posts are in fact the same models who
work at [the club] inflicts the same injury on a consumer irrespective of any
goodwill those models have cultivated in their own “marks,” because that
misconception does not rely upon such goodwill in the first place…. In other
words, the misconception goes to the nature of the product itself, not the
mistaken belief that someone whose imprimatur the consumer values has vouched
for that product.
The district court also correctly held that bad faith
weighed in defendants’ favor. As in Electra, they used third-party
contractors to create the ads and publish them, and there was no evidence that
they ever asked those contractors to use a photo of a specific person.
The district court’s factor balancing was also correct; it
focused on three of the eight factors, discussed above, and then assumed
without deciding that the remaining ones favored plaintiffs. Although that
approach is “discouraged” in this circuit, and to avoid a costly remand
district courts should go through all the factors, it was ok here.
Comment: I’m not really sure how to evaluate this
argument—if it’s often a matter of law, that suggests remand wouldn’t be
necessary in many cases, and if the district court errs in the factors it does
analyze, that seems like it would often make remand necessary anyway, but the
larger problem is, I think, that not all the factors are always relevant. Like
“quality,” for example, which the Second Circuit refuses to go en banc to get
rid of (and, by the way, its rule that you have to march through all the
factors was a later panel rejecting an earlier panel; shouldn’t they have had
to go en banc to do that?) Technically, one could now argue that the
nominative fair use factors should be considered in every Second Circuit case,
at least where raised by the defendant, but that seems useless and perhaps
misdirective where it’s the other factors that are really more relevant.
Anyway, the court concluded, vacatur was not inevitable even
when a district court “neglects to account” for all eight factors. Though it’s
“risky,” in “rare cases … the weight of binding precedent may obviate the need
for a complete Polaroid analysis.” This was such a case, given Electra.
“We cannot fault a district court for its reasonable adherence to recent,
directly-on-point, binding precedent constructed upon substantially
indistinguishable facts.” And plaintiffs didn’t present any reasons to suggest
that the other factors weighed more strongly in their favor than they did in Electra.
Plaintiffs argued that, if more factors favored them than
disfavored, they should have won, but the balancing isn’t a counting exercise
(which, not for nothing, is why the “gotta count them all” analysis is
not great).
False advertising: Lexmark was consistent with prior
circuit precedent that a viable false advertising claim requires the plaintiff
to have been “injured as a result of the misrepresentation, either by direct
diversion of sales or by a lessening of goodwill associated with its products.”
Likewise, Lexmark supported the circuit rule that, although such injury
may be “presumed” from a direct competitor’s “false comparative advertising
claim,” in all other cases, a plaintiff must present some affirmative
“indication of actual injury and causation.” Lexmark didn’t foreclose
courts from granting a presumption of injury to direct competitors while
requiring others to present evidence of injury and causation. There was no
evidence of direct competition, nor was there evidence of injury and causation.
Plaintiffs claimed that they might have lost out on work due
to the “reputational hit” from being linked with a strip club, and that they
were deprived of licensing revenue for their images. The first type of injury
could satisfy Lexmark; there just wasn’t any evidence that it happened.
Even if they couldn’t get direct evidence because people don’t explain why they
don’t hire you, there were other things they could have done: There was nothing
in the record that anyone who might conceivably have hired them ever saw the
posts in question; there was no temporal evidence correlating downturns in
their careers with the appearance of the posts; there was no expert testimony
of “the effect of this kind of R-rated association on a typical model’s career
– much less on these particular models’ careers.”
The second type of injury, lost
licensing revenue, didn’t fall within the Lexmark zone of interests
test. It wasn’t “reputational” injury, and it didn’t flow from the deception
wrought by the ads.
Finally, the district court
correctly found most of the right of publicity claims time-barred. Recent
changes to the NY right of publicity didn’t change the limitations period.
Friday, May 19, 2023
court allows some claims based on allegedly misleading statistical claims for pregnancy test: Bayes' Theorem in the courts
In re Natera Prenatal Testing Litig., No. 22-cv-00985-JST,
2023 WL 3370737, -- F. Supp. 3d – (N.D. Cal. Mar. 28, 2023)
Natera sells Panorama, a noninvasive prenatal testing
(“NIPT”) product which screens for an array of fetal chromosomal and genetic
conditions:
NIPTs are screening tests, not
diagnostic tests; while an NIPT can screen patients for a “high risk” of the
presence of a particular fetal condition, a patient who receives a positive
NIPT result should follow up with diagnostic testing to confirm the presence of
that condition.… Diagnostic tests, though more accurate than NIPTs, are also
more invasive, are associated with a risk of miscarriage, and must be conducted
later in the pregnancy term. In 2020, the American College of Obstetricians and
Gynecologists (“ACOG”) changed its guidance to recommend that all pregnant
persons “be offered both screening and diagnostic testing options.”
Natera’s advertising touted “fewer false positives and fewer
false negatives” and offered patients the ability to “[d]iscover more about
your baby’s health.” It included a testimonial from a patient “now planning a
birth at a regular maternity ward instead of closer to the children’s hospital
thanks to this painless test!” Its website described Panorama as “the most
reliable way of non-invasively assessing a baby’s health,” having been tested
with “the largest prospective NIPT study” with outcomes of “~90% samples with
genetic truth.” It stated that a “high risk” finding for a particular condition
“indicates a very high probability that your baby may have [the] condition.” It
highlighted Panorama’s positive predictive value (PPV) for Down syndrome of
over 90% and describes Panorama as “[n]on-invasive and highly accurate, ...
identif[ying] more than 99% of pregnancies affected with Down syndrome [with]
the lowest reported false positive rate of any prenatal screening test for the
commonly screened chromosomal abnormalities: [Down syndrome], trisomy 18, and
trisomy 13.”
The importance of PPV is easy to overlook. (I’m working on a
paper with Chris Buccafusco now about this problem in music copyright cases.)
If there is any false positive rate at all, and if the base rate of the
tested-for condition is low, then a positive result can be much more likely
than not to be a false positive, even if the test has a very low false positive
rate. And most people find this very difficult to understand—how could a
99% reliable test be wrong more often than not? This is sometimes known as
“base rate neglect.” Down syndrome is the best case for PPV because its
prevalence in the population is much higher than most of the other tested-for
conditions.
As the court explained,
Panorama’s accuracy varies widely
across conditions. While Panorama is very effective in screening for Down
syndrome, it is much less effective in screening for rare genetic conditions,
including those caused by microdeletions. … Natera knew, but did not disclose,
that Panorama has a high rate of false positives for microdeletion-related and
other rare conditions. In 2016, Natera acknowledged the results of a published
study which found that Panorama had an 18% PPV for DiGeorge syndrome, a rare
genetic condition. In other words, 82% of Panorama’s positive results for DiGeorge
syndrome were false positives. Panorama’s PPVs for other rare conditions –
which similarly were not disclosed in marketing materials – are as low as 2-5%,
such that up to 98% of positive results for those conditions are false
positives.
Yet Natera allegedly advertised Panorama “as reliable
overall, prominently emphasizing the accuracy rates of the tests for more
common conditions like Down [s]yndrome [which] Panorama can reasonably detect.”
Natera’s brochure for consumers didn’t include any information about PPV rates,
and the website listed only the PPV for Down syndrome.
The putative class plaintiffs all received Panorama results
requiring further evaluation and subsequently underwent subsequent monitoring
by medical specialists or invasive diagnostic testing, incurring additional
expenses. Each of their Panorama results turned out to be a false positive.
They brought the usual California
statutory claims, warranty and common-law claims, and claims under the
consumer protection laws of other states (Maryland, Illinois, Florida, and New
Jersey).
Claims for misrepresentation by partial omission with
particularity, plaintiffs needed to identify the allegedly misleading
representations that each of them saw and relied upon in deciding to purchase
Panorama, but they didn’t.
However, the court rejected the learned intermediary
doctrine as applied to the fraud-based claims. “Under California law,
failure-to-warn claims brought in products liability actions are subject to the
learned intermediary doctrine, which holds that a manufacturer of prescription drugs
or certain medical devices satisfies its duty to warn by providing adequate
warning to the prescribing physician, rather than the patient.” Natera didn’t
identify any California courts that applied this doctrine to consumer
protection claims. Even if that was sometimes appropriate, Natera didn’t
explain why these claims were “essentially” or “disguised” failure-to-warn
claims, as would be required to do so; plaintiffs were arguing that the
products’ accuracy was misrepresented, not that they posed undisclosed safety
risks. The court reasoned similarly as to other states’ laws.
Did Natera owe a duty to disclose? Under California law, “to
be actionable[,] the omission must be contrary to a representation actually
made by the defendant, or an omission of a fact the defendant was obliged to
disclose.” A plaintiff sufficiently pleads a duty to disclose where: (1) the
plaintiff alleges the omission was material; (2) the alleged defect was central
to the product’s function; and (3) the defendant (a) is plaintiff’s fiduciary,
(b) has “exclusive knowledge” of material facts, (c) “actively conceals” a
material fact, or (d) makes misleading partial representations. Plaintiffs
failed to identify a relevant “defect” in the tests. In another false
advertising case challenging the undisclosed presence of child and slave labor
in the supply chain, the Ninth Circuit rejected the existence of any duty to
disclose because “the labor practices in question ... are not physical defects
that affect the central function of the chocolate products.” Thus, the
omission-based California claims were dismissed with leave to amend. (This
seems to me to be slicing the salami a bit too fine, but I admit I’m
uncomfortable with product liability analogies anyway. The “defect” here is
that the product doesn’t provide the information it claims, which is central to
its function of providing medical information. If it were a physical monitor
that flashed red when it shouldn’t, it seems to me that should count, so why
would it be different here? Also, whether something is a “defect” may depend
entirely on how it’s advertised: if I advertise paint as paint, it’s not
defective, but if I advertise it as a cancer cure it is.)
The court also found that the plaintiffs didn’t need not
plead an independent duty to disclose for omission-based claims brought under
the Maryland, Illinois, Florida, and New Jersey laws.
Implied warranty of merchantability: this requires that
consumer goods, among other things, “are fit for the ordinary purposes for
which such goods are used.” “Plaintiffs plausibly allege that the basic
function of a test which screens for a specified set of genetic conditions is
to accurately detect the risk of such conditions, and that Panorama was unfit
to do so.” But there was no privity as required for an implied warranty of
merchantability, even though plaintiffs alleged reliance on Natera’s
advertising. However, there was a split of district court authority about
whether there’s a third-party beneficiary exception to the vertical privity requirement,
which the court resolved in plaintiffs’ favor.
Plaintiffs alleged they “are the intended third-party beneficiaries of
agreements between Natera and their physicians and health insurers”; that these
agreements to use Panorama “were designed and intended for the benefit of
Plaintiffs ... to make health care decisions,” and that Natera “understood that
Plaintiffs[ ] ... would require that [Panorama] provide reliable and accurate
information regarding genetic abnormalities that may affect their pregnancy and
the well-being of their bab[ies].”
courts continue to jack up materiality requirements; the Lanham Act and the death of common sense?
Delta T LLC v. MacroAir Technologies, Inc., No. EDCV
20-1489-GW-JPRx, 2022 WL 19827572 (C.D. Cal. Nov. 18, 2022)
MacroAir asserted – among other things – a counterclaim for
false advertising under the Lanham Act. Of potential note: Plaintiff aka BAF
(Big Ass Fans) argued that MacroAir needed consumer testimony, consumer
surveys, or expert testimony to establish materiality. The court
stated—contrary to actual practice—that “materiality in false advertising cases
is ‘typically’ proven through consumer surveys,” but immediately qualified this
claim: “that type of evidence is not required.” Still, “[w]hile surveys are not
required, a party advancing a false advertisement claim still must have some
basis for demonstrating a triable issue of fact on the subject,
when-challenged.” This is an example of courts cranking up the liability
standard over time, in the inverse of what they’ve done in trademark cases
where they’ve accepted ever broader theories of liability. Historically,
materiality was usually a matter of common sense; it still should be in
appropriate cases.
The court rejected MacroAir’s attempt to presume materiality
from (alleged) literal falsity. It surveyed the caselaw in the Ninth Circuit,
which was not consistent. Even though materiality is itself not provided for in
the statute—it developed from the injury requirement—the court reasoned that,
in the absence of any statutory guidance, “the elements of a false advertising
claim under the Lanham Act should be treated like the elements of any other
claim which a plaintiff must prove in order to prevail – MacroAir must come
forward with evidence to demonstrate a triable issue of fact on the question.”
Moreover, “it is not the type of advertisement or communication in question in
general that must be material, but the deception itself.”
The court rejected MacroAir’s evidence as insufficient
because of this. Its expert apparently opined on the materiality of
“manufacturers’ claims about warranty, performance, reliability, [ ] safety,
and perceived differences between manufacturers’ offerings in these areas,” but
“that evidence is too broadly-drawn for the false advertising element of
materiality.” Summary judgment dismissing the claim.
copying/explicit references let Roblox proceed with dubious (c) claim; Lego should be watching
Roblox Corp. v. Wowwee Gp. Ltd., 2023 WL 2433970, -- F. Supp. 3d --, No. 22-cv-04476-SI (N.D. Cal. Mar. 9, 2023)
Roblox runs a “digital world where users create virtual
games and experiences and connect with other users.” Users interact with the
platform through virtual characters known as “Avatars.” Roblox’s “Classic
Avatars” are “humanoid figures with cylindrical heads, C-shaped hands,
block-shaped bodies and legs, square or rounded arms, and cartoon-like facial
expressions.” (Comment: That is, they’re just like Lego minifigs.) Roblox
authorized plaintiff Jazwares to manufacture “Avatar Figurines,” real-world
toys based on the digital Avatars. Its TOS provide that users will not use
Roblox content outside of the Roblox Platform, monetize Roblox content, or
imply an association with Roblox for their businesses outside of the Roblox
Platform.
Wowwee sells a line of dolls called “My Avastars,” which
plaintiffs allege were “copied directly from Roblox’s Classic Avatars.” WowWee’s
Vice President of Brand Development & Creative Strategy, Sydney Wiseman,
used her WowWee email address to create a Roblox user account and used her
Roblox account to promote My Avastars dolls on social media, including videos
on her TikTok account. Wiseman narrates, “I was playing roblox and as I was
customizing my avatar I was inspired to create a doll line called my Avastars.”
Defendants allegedly marketed the My Avastars dolls with a “code” that could be
used in the Roblox platform.
Roblox sued for copyright infringement, false advertising,
trademark infringement, false association and false designation of origin,
trade dress infringement, intentional interference with contractual relations,
breach of contract, and false advertising and unfair competition under California
law.
The court found copyright infringement adequately pled as to
several figures, although substantial similarity was a “close issue.” “While
the features for which plaintiffs allege protection are similar to those of
other toys, there are differences including the customizability of features of
plaintiffs’ avatars and the shape of the avatars’ legs.” And defendants’ dolls
were “virtually identical” in shape to Roblox’s avatars.
Looking at the side by side pictures in the complaint, this is a bit hard to swallow, but the evidence of copying/references to Roblox clearly bleed over from the TM side.
| Lindsey Roblox avatar |
| Cindy Roblox avatar |
 |
| Allegedly jnfringing Dreamer My Avastars |
The trade dress claim also survived based on a trade dress definition that Lego surely doesn’t like: “a distinct overall look and feel stemming from at least their (1) humanoid, blocky shape; (2) cylindrical heads; (3) C-shaped hands; (4) block-shaped legs; (5) square or rounded arms; (6) cartoon-like facial expressions and lack of a nose; and (7) the particularized combination of these elements.”
 |
| more images from the complaint |
Interestingly, the contract/tortious interference claims
against US resident defendants had to be arbitrated because of its own TOS. And
Jazwares, Roblox’s licensee, lacked standing for copyright claims, but did have
Lanham Act standing because that doesn’t require copyright or trademark
ownership. “Jazwares has adequately pled injury due to customers associating
the My Avastars dolls with Roblox, leading to lost sales of Jazwares’ Avatar
Figurines.”
court doesn't find consumer protection claim over "sweet cream" plausible without survey; dictionaries insufficient
Sneed v. Ferrero U.S.A., Inc., --- F.Supp.3d ----, No. 22 CV 1183, 2023 WL 2019049 (N.D. Ill. Feb. 15, 2023)
Courts in consumer protection cases reject surveys with abandon when they don't agree with the results, but may also demand them. The court dismissed Sneed’s allegations that Ferrero’s
Kinder Joy eggs were misleading because the label describes the candy as “sweet
cream topped with cocoa wafer bites,” when, in fact, the “cream” is made of
vegetable oils, skim milk powder and whey proteins. Sneed’s argument that
“cream” means a dairy product with a high fat content of at least 18% milkfat relied
on five dictionary definitions and one FDA regulation.
But her complaint recognized the existence of a food
substance known as “artificial cream,” where the milkfat is replaced with
vegetable oils. That meant the question was misleadingness: whether “cream” “has
such a singular and pervasive meaning among the general consuming public that
most consumers believe it to only mean a dairy product with 18% milkfat content
and are therefore likely to be misled by Kinder Joy’s packaging.”
She didn’t successfully allege this. “Although allegations
about the results of consumer surveys are not required as a matter of federal
notice pleading, allegations about how the general consuming public understands
the term ‘cream’ is the kind of thing that makes plausible the conclusory
allegation that ‘sweet cream’ is misleading.” Dictionaries weren’t enough, nor
was it enough that more than half of the package is white and has two large
“drops” of milk and that the front of the package says “sweet cream.” The
ingredient list on the back didn’t include “milk,” “whole milk,” or any other
indication that it is a dairy product with at least 18% milkfat. Plus, there
exist other candies on the market labeled “cream” which are instead made of
artificial cream—Goetze’s “Caramel Creams” (made since 1895), “Cookies ‘n Cream
Bites,” and Twizzlers’ “Filled Twists” that are “orange cream pop” flavor. Dismissed
without prejudice.
if an allegedly falsely advertised product isn't useless, P may have standing to seek injunctive relief
Perez v. Bath & Body Works, LLC, No. 21-cv-05606-BLF, 2023 WL 3467207 (N.D. Cal. May 15, 2023)
Interesting analysis of standing for injunctive relief: Where
the product is a useful one, the court finds standing based on a desire to
purchase it again if truthfully labeled.
Perez alleged that defendant BBW falsely claims that hyaluronic acid, an ingredient in those products, “attracts and retains up to 1,000x its weight in water to make skin look smoother and more supple.” She brought the usual California claims.
BBW argued that there was no standing for injunctive relief because she alleged
that it was scientifically impossible for hyaluronic acid to retain 1,000x its
weight in water. Past cases have said, among other things, that plaintiffs who
“explained that they were not concerned with phosphoric acid, but rather with
whether Coca-Cola was telling the truth on its product’s labels” lacked
standing because their “desire for Coca-Cola to truthfully label its products,
without more, is insufficient to demonstrate that they have suffered any particularized
adverse effects.” Perez alleged that she wanted to purchase BBW products “that
could help improve the appearance of her skin, including, specifically, Bath
& Body Works Hyaluronic Acid and moisturizing products such as those
described above.” But, she alleged, without professional testing or other
expert evidence, she couldn’t determine if BBW was telling the truth about its
products’ features. Even if the formulation or advertising changes, “as long as
Defendants may use inaccurate representations about the capabilities of their
hyaluronic acid products, then when presented with Defendants’ advertising, Ms.
Perez continues to have no way of determining whether the representations
regarding those capabilities are true.”
The court found these allegations sufficient. As the Ninth
Circuit has said, “the threat of future harm may be the consumer’s plausible
allegations that she will be unable to rely on the product’s advertising or
labeling in the future, and so will not purchase the product although she would
like to.” BBW argued that the Ninth Circuit was dealing with wipes that could
conceivably be flushable, but Perez alleged that the claim here was
scientifically impossible. “But the Court declines to look at the threatened
injury so narrowly.” While “a plaintiff must show ‘a sufficient likelihood that
he will again be wronged in a similar way,’ … [a court] ‘must be careful not to
employ too narrow or technical an approach.’ ” The inability to rely on
defendant’s representations was a “similar” injury.
BBW argued that a plaintiff needs to allege a desire to
purchase the product as advertised. But a plaintiff can allege “a concrete,
imminent injury” even without alleging a desire to purchase the product “as
advertised.” Also, this case didn’t involve an allegedly worthless product:
“Even if hyaluronic acid cannot retain 1,000 times its weight in water, it is
not necessarily useless as a moisturizer.”
Why do people fall for pyramid schemes? FTC v. Noland offers examples
FTC v. Noland, 20-cv-00047-DWL (D. Ariz. May 11, 2023)
The decision in FTC v. Noland is notable for its discussion
of the testimony of a number of witnesses supporting the defendant who seem to
have been largely victims of the pyramid scheme found by the court, but
internalized defendant’s messages so strongly that they were unable to see
themselves that way. They often didn’t track expenses or even sales in an
organized way, so they overestimated their net earnings/didn’t notice their net
losses, and seem to have engaged in separate mental accounting of expenses like
attending seminars. They didn’t blame the company for losing money and some
donated large sums to legal defense for the company and its principals. It
gives some depressing insights into how people fall for pyramid schemes and may
stay true believers, thinking only that they themselves have failed to succeed.
Tuesday, May 09, 2023
Apple v. Corellium out: 11th Cir. finds copying for security research transformative
Apple v. Corellium, Inc., No. 21-12835 (11th Cir. May 8, 2023)
The Eleventh Circuit affirmed the core finding that
Corellium’s copying of iOS for security research purposes was fair use, but vacated
and remanded for further analysis of contributory infringement claims and
claims related to the use of the icons, giving Apple another bite at, etc.
I won’t recap the whole opinion, but I do note that the
court of appeals says, perhaps more explicitly than any other court, that the
question is whether a transformative character may reasonably be perceived, not
limiting that formulation to parody:
[T]ransformativeness does not require
unanimity of purpose—or that the new work be entirely distinct—because works
rarely have one purpose. In assessing whether a work is transformative, the
question has always been “whether a [transformative use] may reasonably
be perceived.” Campbell, 510 U.S. at 582 (emphasis added) (finding that a
parody was transformative even though both a song and its parody serve the same
function of entertainment). We don’t ask whether the new product’s only
purpose is transformative.
We’ll see if Warhol makes that obsolete.
Monday, May 08, 2023
Trademark and Unfair Competition Scholarship Roundtable 2023 Reminder: Submissions Due May 15, 2023
The Engelberg Center on Innovation Law & Policy will host this year’s Trademark and Unfair Competition Scholarship Roundtable. The Roundtable is designed to be a forum for the discussion of current trademark and right of publicity scholarship, covering a range of methodologies, topics, and perspectives. Five to six papers will be chosen for discussion over the course of the Roundtable, with each paper allocated an entire hour for discussion and assigned a commentator.
The Roundtable will be held on Friday, October 6, 2023. Participation at the Roundtable will be limited and invitation-only and we expect all participants to have read the papers in advance. The Roundtable will cover the travel and lodging expenses for invited authors.
We invite submissions from academics working on any aspect of trademark, false advertising, marketing, right of publicity, or related areas of the law. Priority will be given to those who can attend the entire event and a dinner the night of the event. Submissions must be of full drafts in Microsoft word format. The deadline for submission is May 15, 2023, and decisions on participation will be made shortly thereafter, ideally, by June 1st.
To submit a draft paper, please fill out the form here (https://cvent.me/8zdYqE) and upload an anonymized version of your draft. Please note that the maximum file size that may be uploaded is 10MB.
For further information about the Roundtable, please email
either: Barton Beebe (NYU): [email protected]; Jennifer Rothman (Penn):
[email protected], or Rebecca Tushnet (Harvard): [email protected].
Wednesday, May 03, 2023
Peloton music library class action fails because consumers probably didn't see the claim
Passman v. Peloton Interactive, Inc., 2023 WL 3195941, No. 19-cv-11711 (LJL) (S.D.N.Y. May 2, 2023)
Interesting discussion of the way in which the objective
reasonable consumer standard allows consumers classes to bring certain probabilistic
claims, where some consumers might have different interpretations, although the
court ultimately denies certification because of damages/price premium issues.
Plaintiffs’ claims under New York law were based on the
offer of an “ever-growing” or “growing” library of live and on-demand studio
classes, which offer was allegedly false because a bunch of classes were pulled
from the library because of unlicensed music use. After it was sued in 2019, Peloton
removed approximately 6,500 on-demand classes from its library, leaving
approximately 7,000 classes available to its members.
The court initially held that problems with plaintiffs’
expert’s conjoint damages model went to weight rather than admissibility, since
it was a model that was consistent with their theory of liability.
Materiality and falsity were common questions subject to an
objective inquiry into how a reasonable consumer would react. Peloton argued that
there was too much variation in how, when, and why consumers bought subscriptions.
“Defendant’s argument confuses the question of whether a reasonable consumer
would likely be misled by an allegedly false advertisement with the separate
question—relevant where reliance is at issue—of whether an individual consumer
was misled by the advertisement…. The
inquiry demands an objective analysis of the understandings a reasonable
consumer would draw from a challenged statement, not the significance of that
challenged statement to an individual consumer’s purchasing decision.” While
context is relevant to that, it’s ad context, not any possible context:
[W]hat is relevant to the analysis
is not the number of products purchased, their exact identity, or the
circumstances under which they were purchased; what matters is the nature of
the allegedly false statement and how consumers interact with the false
statement and therefore understand it. Thus, courts generally have no
difficulty finding named plaintiffs typical of a class so long as the
challenged statement is consistent across the class.
Peloton also argued that falsity wasn’t a common question
because there wasn’t 100% agreement in plaintiffs’ survey on the meaning of “ever-growing”—the
survey found that only 76.5% of respondents thought that “ever growing” meant
“increase over time.” But misleadingness is an objective inquiry. “Evidence
that actual consumers, in fact, interpreted the challenged statement in line
with ‘the plaintiffs’ proffered theory of deception’ is relevant to, and may be
necessary for, the ultimate conclusion that a reasonable consumer would have
been deceived.” But “the fact that
consumers may have interpreted the statement differently does not preclude
certification.” Differences in understanding might bear on injury, but falsity
itself “cannot differ from case to case or be based upon whether the case is
prosecuted on an individual or a class basis; it turns upon an objective
analysis that applies across cases.”
Nonetheless, because causation, injury and damages weren’t
common among the putative class members, individual questions predominated and
a Rule 23(b)(3) class couldn’t be certified. The evidence submitted didn’t show
a price premium or propose a methodology which could be used to demonstrate a
price premium.
Peloton presented affirmative evidence that there was no
price premium and “compelling evidence” that plaintiffs failed to carry their burden
on predominance. The challenged claim wasn’t on a product label or package:
[N]o purchaser of a Peloton product
need have been exposed to the Challenged Statement and the evidence suggests
that many of the purchasers were not exposed to the Challenged Statement. The
Challenged Statement did not appear in every Peloton advertisement and did not
appear on all of Peloton’s marketing materials. Rather, the Challenged
Statement appeared in a relatively small subset of Peloton’s advertisements, and
did not appear in any television advertisements, which represented Defendant’s
largest advertising channel during the Class Period. The Challenged Statement
also appeared on only four of the 269 pages of Pelton’s website, the primary
place where consumers purchase Peloton products.
Even when it did appear, the claim wasn’t alone or the most
prominent. E.g.: “Experience unlimited access to the world’s best instructors
anytime, anywhere, with 15+ daily live classes and an ever-growing library of
9,000+ classes available on-demand.”
Website tracking data suggested that, at most, 10.99% of
website visitors could have been exposed to the statement, but that didn’t show
that they actually saw/noticed it.Peloton’s expert did a survey about whether
consumers even noticed the claim. She found that only 0.7%-1.3% of the test
group noticed the statement and that there was no statistically significant
difference between the test and control with respect to their conclusions about
the Peloton products after viewing the webpages. And, despite its statements
above, the court also gave weight to plaintiffs’ own survey that showed that
people ascribed “different meanings”: while 76.5% understood that the number of
classes in the Peloton library would increase over time, 61.8% of them expected
the number of classes to increase because no classes would be removed from the
library, while 31.4% believed that more classes would be added than removed.
Also, 79.8% interpreted the statement to mean the number of classes would
increase each day, week, or month, while 3.7% thought the increase would be
annual (the remainder had no view). This wasn’t fatal: “materiality is an objective
inquiry and thus subject to common proof.”
But this evidence does bear on
whether the Challenged Statement could have caused a price impact. That many
had differing views over what the Challenged Statement meant and took different
meaning from it suggests both that the statement did not have a powerful
marketing impact and that those who saw the Challenged Statement may not have
interpreted it in such a way as to give rise to a price premium.
In addition, the price of each relevant Peloton offering
remained constant both before, during, and for almost eighteen months after the
class period. While those results “could perhaps be explained away if there
were other confounding factors, including if Defendant offered something
additional of value to consumers after the takedown or if sales fell markedly,”
Peloton’s expert looked for other confounding factors and found none. The
burden of demonstrating that there was a price premium (and thus that the
predominance requirements of Rule 23(b)(3) have been met) was plaintiffs’ and
they did not satisfy it. Plaintiffs’ conjoint model assumed that individuals
saw and noticed the challenged statement to generate its price premium
calculations, but that assumption wasn’t supported by the evidence. Plaintiffs’
survey also didn’t distinguish the value of Peloton’s library of classes—a Peloton
innovation—from the value of its purported “ever-growing” size.
In addition, plaintiffs didn’t show that there was a model
capable of measuring the damages attributable to their theory of liability,
since their model conflated the existence of a library with its ever-growing
size. “Courts routinely reject price premium methodologies under Comcast when
the proposed methodologies do not attempt to isolate the premium due only to
the allegedly misleading marketing statement.” The model also didn’t consider
the impact of supply-side factors. “Because Defendant might have responded to
the decreased demand by producing fewer Peloton products, the damages of the
putative class members would presumably be less.” Nor did the model “consider
how competitors would have reacted to a decrease in demand for Peloton
products, which could in turn affect the supply of Peloton products.” It would be
different if the products had only one relevant attribute and that attribute
was falsely advertised, since there the entire price paid would be based on
falsity and no price premium analysis would be required. [The hypothetical is a
joint pain cream that doesn’t cure joint pain; note that this is not a
one-attribute product, since there are non-cream treatments for joint pain, so
in fact there are at least two attributes, one of which is true in the hypo.]
The same flaws doomed plaintiffs’ omission theory, which
relied on a consumer survey showing that 48.9% of respondents would be either
extremely or moderately concerned if Peloton were forced by legal action to
remove 50% of their classes. Peloton’s designated witness testified that there
were only “a single-digit number of subscription cancellations that were
directly attributable to ... the on-demand classes removed from [Peloton’s]
library in March 2019.”
Second Circuit finds "therapeutic grade"/physical effects claims for essential oils falsifiable; suggests that lack of substantiation violates NY law
MacNaughton v. Young Living Essential Oils, LC, 2023 WL 3185045, No. 22-0344, -- F.4th -- (2d Cir. May 2, 2023)
In 2020, NAD found that Young Living’s claims that its essential
oils are “therapeutic-grade” and impart physical and/or mental health benefits were
“unsupported.” But MacNaughton had already spent money on Young Living’s
products, including lavender oil advertised to “promote[] [a] feeling of calm
and fight[] occasional nervous tension” and peppermint oil that allegedly
“helps to maintain energy levels.” Feeling misled by claims that the product
would have effects like “promot[ing] feelings of relaxation & tranquility,”
MacNaughton sued under common law and various state statutes, including NY’s
GBL. The district court claims that its
products would do things like “help[] to maintain energy levels” was
run-of-the-mill puffery.
Relying on Int’l Code Council, Inc. v. UpCodes Inc., 43
F.4th 46 (2d Cir. 2022), the court of appeals reversed, though it did affirm
the dismissal of warranty claims.
Young Living instructs its salespeople that in “describing
therapeutic-grade oils,” they should mention that “every essential oil . . .
has the highest naturally-occurring blend of constituents to maximize the
desired effect.” The website also formerly contained a statement that though
the therapeutic-grade “promise” was “bold,” the salesperson could “share [the]
products with confidence, knowing that Young Living truly has the experience to
produce essential oils that work.” Similar guarantees remain on Young Living’s
“various blogs and other websites.” Young Living continued to advertise the products
as being “therapeutic-grade.” MacNaughton cited three studies, all of which
conclude there is insufficient evidence to find that aromatherapy is an
effective treatment of anxiety or of any other type of condition.
The breach of warranty claims were properly dismissed
because MacNaughton failed to allege proper notice and privity of contract.
Puffery comes in two forms (1) subjective statements that
cannot be proven true or false and are therefore non-actionable puffery as a
matter of law and (2) objective statements that can be proven true or false but
are so exaggerated that no reasonable buyer could justifiably rely on them.
Under category one, claims that a website designed to
compile construction codes “provides a complete understanding of relevant
material” and that the author of a book on animals “thoroughly researched
dozens and dozens of animals” have been deemed non-actionable puffery as a
matter of law. Under category two, claims can be falsifiable but “so patently
hyperbolic that any allegations that it misled consumers are facially
implausible,” such as a bubblegum brand advertising that its gum permits
chewers to “blow a bubble as big as the moon.” “Yet, if the company falsely
advertised that you could ‘blow a bubble bigger than your own head,’ it is
plausible that a reasonable buyer could be misled.”
“Once the statement is identified as both provable as false
and plausible, a defendant can only prevail on the puffery defense after a
fact-intensive inquiry on how a reasonable buyer would react. That inquiry
cannot be resolved at the pleadings stage.” That was the case here. Young
Living’s statements about its “therapeutic-grade” oils having health and
medicinal benefits are both provable and not “so patently hyperbolic that any
allegations that it misled consumers are facially implausible.”
“Therapeutic-grade” was “not a subjective or vague term, but
rather one that represents the item possesses a degree of quality as to produce
healing.” It was distinguishable from grade + “adjectives that are merely
general representations of superiority” such as “superior grade” or “prime
grade.”
The ad context was also relevant:
Along with the “therapeutic-grade”
label, Young Living also promised that each Product would produce particular
medicinal or physical effects, such as “promot[ing] a sense of clarity and
focus.” Additionally, Young Living
directed its salespeople to emphasize that every oil “has the highest
naturally-occurring blend of constituents to maximize the desired effect” and
that “Young Living truly has the experience to produce essential oils that
work.” The accuracy of all these statements and claims is provable. ‘’
NAD/NARB rulings weren’t binding, but were relevant to the plausibility
of deception of a reasonable consumer. These claims were all provable, and they
weren’t patently hyperbolic. Thus, puffery couldn’t be resolved on a motion to
dismiss.
In addition, the court rejected Young Living’s argument that
the plaintiff alleged only lack of substantiation, not falsity. Notably, “Young
Living does not cite any binding case law to support its argument that the New
York General Business Law does not protect against advertising that lacks
substantiation.”
Unjust enrichment was also sufficiently pled.
Tuesday, May 02, 2023
Pandemic art kit didn't infringe artist's rights
Keck v. Mix Creative Learning Center, LLC, No. 4:21-CV-00430, 2022 WL 19691177 (S.D. Tex. Dec. 19, 2022)
Technically, the trademark analysis here is weird (the
parties agreed that the copyright fair use analysis would determine the
trademark fair use analysis; the plaintiff’s lawyer is Higbee, FWIW), but the
trademark claim is so clearly Dastar-barred and parasitic on the
copyright claims that it’s hard to object.
Keck is a mixed-media artist who registered a trademark in
her name (the court doesn’t specify for what, which is an indication of the
level of attention given the trademark claims). Defendants used a couple of
pictures of her Dog Art work as an example of a style that kids using its art
kits could emulate. The court found that this was fair use and granted summary
judgment to defendants.
During the pandemic, defendants (a local art studio and its
principal) began selling art kits online “so that the students could learn
about media, styles and techniques that world-renowned and lesser-known artists
use, as inspiration for the students to create their own works of art.” According
to Defendants, “[t]he artist’s biography and a sample of the artist’s style of
work was included in each kit to recognize the artist and to document and teach
the particular art style, as well as recognize such works as part of historic
scholarship, promote discussion and criticism, and to inspire each student to
create their own masterpiece works.” The kits included slides “with publicly
available images of the artist’s works along with historical and biographical
information about the artist from, for example, the artist’s publicly available
website.” Defendants provided zoom art classes to accompany the kits. As part
of these lessons, the individual defendant described the artists and their
techniques. Several artists featured in the kits applauded and even reposted
the children’s work on their Instagram accounts.
One such kit was the “Michel Keck inspired dog masterpiece kit.” The kit consisted of pictures of six pieces of Plaintiff’s art, biographical slides, and materials such as paint, paint brushes, and collage-style puzzle pieces “so that the students would have the necessary materials to create their own masterpieces in the form of mosaics and/or completely new artworks.” The copies of Keck’s work were made by right-clicking Google images.
 |
| defendant's ad for art kit |
Defendants sold only six of these kits—including two sold to Keck—for gross revenue of $240. Once they received notice of the lawsuit, they removed all art kits from the website. Plaintiff continued to demand $150,000 per work, which can’t have helped the optics.
The use was commercial but transformative. “While Defendants made no alterations to the works, they included the pictures in a kit with tools to allow students to create their own art. Defendants also prepared slides with biographical information on the artists.” Unlike in Warhol v. Goldsmith, the purpose and character was “not to create commercial visual art but to teach children about different artistic styles…. [T]he use of source material, accompanied by biographical information or other scholarship or lessons, transforms art into an educational tool.”
The court noted that defendants weren’t “in the business of selling artistic reproductions and did not sell these kits as works of art themselves. Defendants did not include Keck’s art in the kit for their inherent decorative value but to demonstrate a specific style of art.” The educational purpose didn’t need to be “as rigorous as that provided in a museum exhibit or university art history course.” Defendants also acted in good faith. “They had no warning before this of Plaintiff’s—or any other artist’s—displeasure.”
Interestingly, this all only tilted factor one “slightly” in favor of defendants.
Nature of the work: favored plaintiff, but least important.
Amount/substantiality: not important; six pieces of art were used (the court doesn’t discuss size or resolution).
Market effect: The proper market is not “licensing of any kind” or “merchandising.” “Conceptualizing a defendant’s conduct too broadly would render any unauthorized as harmful to the market for an artist’s work, calling into question the usefulness of this fourth factor.” The proper market was licensing for art kits that use the art as “an example of a particular art style and inspiration for students.” This didn't show market harm. Moreover, “[t]he widespread use of Plaintiff’s art for educational lessons would likely, if anything, increase her name recognition and commercial value.”
"same" claim was literally false where probiotics had different strains and different profiles
ExeGi Pharma, LLC v. Brookfield Pharmaceuticals, LLC, ---
F.Supp.3d ----, 2023 WL 3142311, No. 20-CV-192-JPS (E.D. Wis. Mar. 21, 2023)
ExeGi sued Brookfield for state and federal false
advertising/tortious interference. The court here resolves only the Lanham Act
claims, partially in ExeGi’s favor.
The parties compete in the market for probiotics. ExeGi
sells Visbiome; Brookfield sells HPP as a food product. “To the extent HPP is
marketed as a medical food and deemed a medical food, it is required to be
consumed under the supervision of a physician.” Brookfield attempted to copy
Visbiome’s formulation based on its expired patent; HPP is produced in a
different manufacturing facility than Visbiome, under different conditions, but
was made with the same species of bacteria as were listed on the expired patent
for the formulation of Visbiome plus an additional species of bacteria. Prior
to the expiration of the patent, however, three of the strains listed in the
patent were reclassified as different strains from different species. “Thus,
HPP has three species of bacteria not in the formulation of Visbiome, and
Visbiome has one species of bacteria not in the formulation of HPP.” Also of
relevance, “[d]ifferent strains of bacteria within the same genus and species
can have different functionalities in and benefits to the human body, and can
have widely differing performance characteristics and modes of action.… HPP and
Visbiome have sufficiently different metabolic profiles, meaning the two
products are … genetically and biologically different.”
Visbiome “is one of the most extensively studied probiotics
on the market, having been the subject of more than 70 human clinical trials.”
Brookfield “formulated” HPP “as a generic to” Visbiome. Its
label doesn’t list the exact quantity of each bacteria species or strain
designation, noting instead that its blend of bacteria is proprietary. The
label indicates that HPP is a medical food for the dietary management of
dysbiosis associated with gastrointestinal conditions such as irritable bowel
syndrome and ulcerative colitis. It doesn’t explicitly claim to have been the
subject of tests or studies. The insert says:
High Potency Probiotic Capsules are
labeled as a medical food as defined by the Orphan Drug Act and additional FDA
regulations. … This medical food is not subject to NDA or ANDA approval and is
not an Orange Book product. This product is intended to be used under active
medical supervision.
These statements have not been
evaluated by the Food and Drug Administration. This product is not intended to
diagnose, treat, cure or prevent any disease.
INDICATIONS AND USAGE
High Potency Probiotic Capsules are
intended for the dietary management of individuals with distinct nutritional
requirements relating to dysbiosis associated with GI conditions such as IBS
and [ulcerative colitis]. High Potency Probiotic Capsules are a non-drug
medical food that addresses distinct nutritional requirements to promote
microbial balance in people with dysbiosis associated with IBS that cannot be
achieved by modification of diet alone.
Maintenance of a healthy gut
microbiota contributes to an overall healthy gastrointestinal environment.
[citations]
Brookfield relied upon a summary of scientific literature to
support its dietary management claims, along with dossiers created for each of
the bacteria used in HPP. No clinical trials have been performed on the HPP
formulation as a whole. With regard to the nine individual strains of bacteria
contained in HPP, there have been no clinical studies performed on five of
those strains.
It also made a number of comparative claims that it was
generic, comparable, comparable generic, compared to, competed against, had the
“same strains,” had the “same probiotic bacteria,” and had the same GCN as
Visbiome (a GCN is a standard number assigned by a drug pricing service to
drugs). Although there don’t seem to have been surveys on this, “[m]ost of the
public and some clinicians incorrectly refer to genus and species as ‘strains.’”
Brookfield identified its primary customers for HPP as “the
large pharmaceutical wholesalers” and retail pharmacies, the latter using
rebate programs that typically involve incentivizing one choice over another
generic. It didn’t advertise directly to
clinicians or consumers, aside from the product label and package insert.
Brookfield represented in communications directly to
individual retailers that it has the “same strains” of bacteria as Visbiome. A Brookfield
witness clarified that this statement was not intended to mean that the
products contained “all of the same strains,” but rather “many of the same
strains.” Its scripted answer to questions regarding similarities is: “Brookfield’s
High Potency Probiotic Capsules contain the same probiotic bacteria in the same
total potency per capsule (112.5 billion bacteria) as (Visbiome). Since precise
formulas are proprietary, we are unable to make an exact comparison to (Visbiome).”
First Databank, the largest of the drug compendia, groups
drug and non-drug products under the same GCN if they have the same active
ingredients, dosage form, route of administration, and strength. The parties’
products were at one time given the same GCN code, but that is no longer true. Brookfield
initially represented to First Databank that HPP contains eight probiotic
strains, rather than the nine it actually contains, which was corrected in 2019,
when a revised label with nine strains was submitted. Brookfield also represented
to First Databank that its product was a “Generic Equivalent” of VSL #3 and
Visbiome and a “medical food.” It made similar statements to other drug compendia
and wholesalers.
Brookfield’s witness testified that listing VSL #3 and
Visbiome as products to which HPP is a “generic equivalent” on the HDA form
“point[s]” recipients of the form “in the right direction of what these
products are similar to,” though it was not accurate to refer to HPP as a
generic equivalent of Visbiome because that term only has meaning in a drug
context. ExeGi’s expert also stated that this was inaccurate because HPP and
Visbiome are “genetically [and] biologically ... different.”
Unlike a drug, a medical food cannot claim to prevent,
treat, cure or mitigate the symptoms of a disease without making a drug claim
that would require FDA approval. The statute defines a medical food as “a food
which is formulated to be consumed or administered enterally under the
supervision of a physician and which is intended for the specific dietary
management of a disease or condition for which distinctive nutritional
requirements, based on recognized scientific principles, are established by
medical evaluation.” But what constitutes an established “distinctive
nutritional requirement” is not specifically defined by the FDA. The FDA
inspected the facility that was manufacturing HPP, and they requested HPP’s
label and packaging insert. It has not initiated any enforcement action as a
result of its investigation.
The FDA’s Orange Book provides therapeutic equivalence
evaluations, but it doesn’t apply to medical foods.
Did the non-label/package statements qualify as “commercial
advertising or promotion”? Yes:
Brookfield disseminated information about HPP to the three largest
pharmaceutical wholesalers, as well as directly to individual retailers. These communications
were more than “purely negotiation of individual contracts, but rather
encompass a variety of media through which Brookfield represented information
about HPP.”
FDA/FDCA preclusion: ExeGi argued that it wasn’t asking the
court to rule on whether HPP was actually a medical food, but whether
Brookfield held HPP out as a medical food “despite having no data upon which to
make such claims and affirmatively determining not to gather such data.” That
didn’t avoid preclusion: “analyzing the quality of the data proffered by
Brookfield in support of its claim that HPP is a medical food—or even
determining that there is no data at all, as ExeGi would have the Court
find—would require it to analyze an area that the FDA has expressly held out as
its own for rulemaking.” Summary judgment granted to Brookfield as to this
aspect of the case.
Literal falsity of the other statements: The court rehearsed
the Seventh Circuit’s rather incoherent treatment of literal falsity, including
the challenging statement that “‘literal’ must be understood in the common
colloquial sense in which Americans ... say things like ‘I am literally out of
my mind.’ ” Consistent with the Seventh Circuit’s, let’s say confident,
approach to judicial factfinding, the court found literal falsity as to “same”
claims but not “generic” claims.
Brookfield argued that “same” claims weren’t literally false
because, in context, it didn’t mean
“all of the same strains,” but rather “many of the same
strains.” The Court disagreed. Dictionaries define “same” as “identical or
equal; resembling in every relevant respect” and “resembling in every relevant
respect; being one without addition, change, or discontinuance.” The products
aren’t the same in that way. “[D]ifferent
strains of bacteria within the same genus and species can provide different
benefits to the human body, function differently in the human body, and have ‘widely
differing’ performance characteristics and modes of action, as well as ‘sufficiently
different’ metabolic profiles.” [Whose burden is it to show that “can” is “do”
here?] Visbiome has also been the subject of over 70 human clinical trials,
while HPP has not. “Same” was thus literally false. Even if Brookfield meant to
say the “same species” instead of the “same strains,” that statement would
still be literally false.
Brookfield argued that “same” didn’t mean “all the same,”
but it required the court to read “same” as “many of the same” or “some of the same.”
This
defies common sense. In the Court’s
view, the word “same” unambiguously and unequivocally means “identical” or
“equivalent” and, based on the undisputed evidence before it, “could not
reasonably be understood to mean anything different.”
Generic/generic equivalent: There was a disputed issue of
material fact on whether “generic” or “generic equivalent” had to mean “identical”
in the context of non-FDA-approved medical foods. Brookfield noted that ExeGi
hadn’t performed any testing to disprove genericity or evidence about the
meaning of these terms in the context of non-drug products that are not subject
to FDA approval. The parties offered conflicting evidence on whether “generic”
meant “can be used as a substitute,” could apply to products with different strains,
meant “lower-cost alternative of a similar product,” etc. But Brookfield didn’t
show any relevant industry evidence to support its definition of “generic
equivalent,” and its own testimony showed that it didn’t consider that HPP was
a generic equivalent to Visbiome (it argued that it had used the phrase on a
form that presumed FDA-approved drug status and was trying to fit a square peg in
a round hole), so that was literally false:
The undisputed evidence indicates
that industry players view the term “generic equivalent” as meaning a “substitute”
for or an “equivalent” to another product. Thus, the undisputed
evidence—particularly that products with different strains of bacteria within
the same genus and species can have widely differing effects—makes it wholly
clear to the Court that calling these products “generic equivalents” is
literally false.
Materiality: Materially requires only a likely effect, not
an actual effect, on consumers, so disputes over whether the challenged statements
actually influenced drug compendia’s, wholesalers’, retailers’, or ultimate
consumers’ decisions as to HPP compared to Visbiome were immaterial. Factors
that go to materiality of a representation include “(1) consumer motivation,
which typically considers the importance of the product or service feature to
which a misrepresentation is directed; (2) consumer reliance, which considers
how a misrepresentation is used; and (3) consumer concern, which considers the
extent to which a misrepresentation departs from the facts.”
The challenged statements were material, given Brookfield’s
marketing goal to get the same GCN as Visbiome. It was irrelevant that drug
compendia relied only upon the labels of the products, and not the form
claiming “generic equivalence,” in assigning GCNs. “This fact is immaterial
given the legal standard of capacity to influence choice, rather than actual
influence. While evidence of actual influence is a factor, it is not
dispositive.” [Interesting interaction with Article III standing, it seems to
me.] Emails from customers inquiring whether Visbiome is the “same” as HPP,
among other evidence, showed that equivalence was “a product feature of concern
to consumers.”
Actual/likely injury: ExeGi showed evidence of sales
diversion, including customer statements explaining that they wanted to buy
Visbiome but were sold HPP instead, including one customer who complained to
ExeGi that a pharmacist informed her that HPP was the same as Visbiome and
FDA-approved, both of which were literally false.
The common-law unfair competition claim received the same
treatment; tortious interference with contract had genuine factual issues that
precluded summary judgment.
The court also found permanent injunctive relief appropriate.
“[T]he scope of ExeGi’s proposed permanent injunction would not remove HPP from
the market; it would address only cessation of the challenged statements” that
the court found literally false.