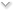Pacira Biosciences, Inc. v. Nephron Sterile Compounding Center, LLC, No. 3:23-5552-CMC, 2024 WL 3656489 (D.S.C. Jul. 15, 2024)
Pacira, which sells non-opioid pain management products, including
Exparel, sued Nephron for false advertising. Exparel is “bupivacaine suspended
in multivesicular liposomes,” and is injected at a surgical site during or
shortly after surgery to manage and reduce post-surgical pain.
Nephron allegedly operates a compounding pharmacy that
compounds BKK, comprised of ketorolac, ketamine, and bupivacaine in a syringe
for combined use, and RKK, a compounded drug consisting of syringes of
ketorolac, ketamine, and ropivacaine for combined use.
Compounded drugs are not FDA-approved, but may be made and
sold under certain circumstances, including rules about outsourcing facilities,
which may not compound using bulk drug substances unless the bulk drug
substances are on a relevant FDA list of clinical need/shortage drugs.
Pacira alleged that the production of BKK and RKK was not covered
by the FDCA’s protection for compounded drugs because (1) BKK and RKK do not
appear on the FDA’s drug shortage list, and (2) the bulk drug substances from
which BKK and RKK are made do not appear on the FDA’s list of bulk drug
substances for which there is a clinical need. But, of course, the FDCA may not
be enforced by private parties, so Pacira turned to the Lanham Act.
Thus, Pacira alleged that defendants “have engaged in a
sustained campaign to promote their drug cocktail products as safe and
effective opioid alternatives through demonstrably false and misleading
advertisements – including blatantly false statements that their drugs are
safer and more effective than EXPAREL.” They also allegedly claimed or implied
that BKK and RKK compounds have been approved by the FDA and/or subjected to
clinical studies and trials.
 |
| Caption: Nephron is fully inspected and approved by the FDA! |
 |
| Footer with FDA approved logo |
Nephron argued that its website was not false: The allegedly deceptive statements, a “Nephron is fully inspected and approved by the FDA!” banner and “FDA APPROVED” logo at the foot of Nephron’s website appeared in the same place on every page of Nephron’s website, including its landing page. It argued that, in context, the banner and footer, which included other logos, such as “MADE IN U.S.A.,” obviously referenced only Nephron, and Nephron is, in fact, a registered 503B outsourcing facility both certified and regularly inspected by the FDA. Anyway, it argued, implicit misrepresentations of FDA approval weren’t actionable.
Pacira responded that (1) the FDA doesn’t approve facilities,
(2) the statements would be attributed to BKK and RKK’s FDA approval, (3)
customers looking for information on those specific products wouldn’t
necessarily peruse every page to see what repeats, and (4) “Made in the USA” is
the kind of statement that consumers would attribute to the products, not
the facilities, so the footer logos encouraged confusion rather than diminished
it.
Despite the plausibility of these arguments, the court
adopted the rule that “the law does not impute representations of government
approval ... in the absence of explicit claims.”
 |
| example of product benefit claims for pain, complications, etc. |
 |
| slide specifically claiming superiority to Exparel and identifying it as "competition" |
Allegedly false claims to hospitals and providers in presentation and other marketing materials about the efficacy, safety, and superiority of BKK and RKK: First, it was plausible to attribute those to Nephron because it hired the person who created the slide show and conducted the presentations. He allegedly “not only developed advertisements and marketing materials for BKK, but he also actively participated in sales pitches and other promotional events nationwide to sell.” This was enough at the pleading stage to impute his actions to Nephron. However, the same “implicit misrepresentation of government approval” rule applied to FDA-approval-related claims. But Pacira also alleged that claims of improved patient safety, satisfaction, recovery time, outcomes, and patient experience were false and misleading. It also alleged that Nephron’s superiority claims, including that BKK and RKK are more “efficacious for long term analgesia” and “post operative pain” than Exparel were literally false. At this stage, the allegations were sufficient as to the safety, efficacy, etc. statements.
 |
| footer claiming that Nephron is a 503B outsourcing facility |
 |
| 503B outsourcing facility claim as part of Nephron logo |
Somewhat puzzlingly to me, the court also allowed claims based on the idea that the logo indicating Nephron is a 503B outsourcing facility conveys the false impression that BKK and RKK products are produced by a 503B-compliant facility. A facility isn’t compliant if it compounds drugs it shouldn’t, and Pacira alleged that this was the case. “Nephron’s claim it is a 503B outsourcing facility, even if true, could falsely imply BKK and RKK satisfy the requirements of § 353b, if, indeed, they do not.” Pacira also alleged reasonable consumer reliance on the misrepresentation by alleging that, “[o]n information and belief, healthcare providers and consumers have reasonably relied on Defendants’ false and misleading statements when deciding to purchase BKK or RKK instead of EXPAREL” and that if they’d known the truth, they wouldn’t have bought the drugs.
What about “commercial advertising or promotion”? Nephron
objected that Pacira didn’t define the relevant market or allege to whom the
presentations were disseminated. First, the statements were commercial speech
promoting Pacira’s products that were provided to a relevant market –
healthcare providers. But were such “product overview” statements in presentations
just medical education? No; “it would strain credulity to find Nephron did not
intend to turn a profit convincing its target audience to purchase BKK and RKK.”
Pacira sufficiently alleged injury.
Were the Lanham Act claims precluded by the FDCA? Nephron
argued that it could only be found to be falsely advertising if the court
interpreted the FDCA and determined that it was violating the compounding
regulations, but that interpretation/determination is for the FDCA. [Side note:
does this argument work in an age of lack of deference to agencies? Especially
if the question is what conduct satisfies the legal standard set out in the law?
Without Chevron, is a decision really committed to the FDA, or to a
court? I think I just found an interesting student note topic.]
Pom Wonderful LLC v. Coca-Cola Co., 573 U.S. 102 (2014), provides
the governing law. [This isn’t really correct—Pom involved a deception
theory that didn’t rely on the FDA’s rules and Coca-Cola argued that its
compliance with FDA’s rules precluded the deception theory. Here, the deception
theory does rely on the FDA’s rules.] The court here relied on Pom’s
policy-based reasoning: The FDA is for health and safety, not primarily
consumer protection; the Lanham Act is primarily about consumer protection. The
FDA lacks expertise in assessing whether people are deceived. [Again, while I’m
substantively in sympathy with Pacira on the policy, that may be true—but the
FDA is the expert on whether compounding facilities are complying with
its rules, which is factual key to this specific theory of deception.] Thus,
while characterizing defendant’s conduct as “illegal,” “unlawful,” and posing
“significant risks to patient safety and health” in the complaint was “overzealous”
because it could “implicate the need for enforcement by the FDCA,” the gravamen
of Pacira’s allegations were falsity and misleadingness and resulting harm to
Pacira.